Welcome to Tangshan Moneide Trading Co., Ltd.

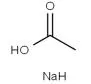
use of coumarin
Introduction to 4-Methyldiphenylamine: A Critical Pharmaceutical Intermediate In the complex landscape of pharmaceutical and specialty chemical synthesis, the purity and reliability of intermediates are paramount. 4-Methyldiphenylamine stands out as a vital compound, playing a crucial role as a `drug substance intermediate` in the production of various active pharmaceutical ingredients (APIs) and fine chemicals. Its unique chemical structure and reactivity make it indispensable across multiple synthetic pathways, impacting the efficacy and safety of final drug products. Moneide Chem specializes in delivering high-purity 4-Methyldiphenylamine as part of our comprehensive portfolio of `bulk drugs and intermediates`, ensuring pharmaceutical-grade quality for our global partners. The demand for high-quality intermediates like 4-Methyldiphenylamine is consistently growing, driven by advancements in medicinal chemistry and stringent regulatory requirements. As pharmaceutical companies strive for greater specificity and reduced side effects in their drug formulations, the quality of starting materials directly correlates with the success of their R&D and manufacturing efforts. Our commitment at Moneide Chem is to provide 4-Methyldiphenylamine that meets and exceeds international standards, offering exceptional batch consistency and a robust supply chain to support critical projects. Industry Trends & Market Dynamics for Pharmaceutical Intermediates The global market for `bulk drugs and intermediates`, including compounds like 4-Methyldiphenylamine , is characterized by several key trends. Firstly, there's an undeniable shift towards more sustainable and green chemistry practices. Manufacturers are increasingly seeking intermediates produced through environmentally friendly processes, reducing solvent use, energy consumption, and waste generation. This trend is not merely regulatory-driven but also reflects a growing corporate responsibility. Secondly, supply chain resilience has become a paramount concern, particularly after recent global disruptions. Companies are diversifying their sourcing strategies, looking for reliable partners with robust manufacturing capabilities and geographic flexibility. This has led to an increased emphasis on transparent supply chains and comprehensive quality control from raw material to finished intermediate. Thirdly, technological advancements in synthetic methodologies, such as continuous flow chemistry and biocatalysis, are revolutionizing the production of intermediates. These innovations promise higher yields, improved purity, and reduced operational costs, driving down the overall cost of drug manufacturing while maintaining or enhancing quality. Moneide Chem actively invests in research and development to incorporate these cutting-edge techniques into our production of 4-Methyldiphenylamine , ensuring our clients benefit from the most efficient and advanced manufacturing processes available. Technical Specifications of 4-Methyldiphenylamine Understanding the precise technical specifications of 4-Methyldiphenylamine is critical for its effective integration into complex synthetic schemes. Our product is manufactured to exacting standards, ensuring high purity and consistent physiochemical properties. Key parameters such as molecular weight, melting point, assay purity, and impurity profile are rigorously controlled and documented. Product Specification Table: 4-Methyldiphenylamine Parameter Specification Testing Method Chemical Name N-(4-methylphenyl)aniline; 4-Methyldiphenylamine N/A CAS Number 637-04-7 N/A Molecular Formula C₁₃H₁₃N N/A Molecular Weight 183.25 g/mol Calculated Appearance White to off-white crystalline powder Visual Inspection Purity (HPLC) ≥ 99.0% HPLC-UV Melting Point 89-92 °C DSC Loss on Drying ≤ 0.5% KF Titration/Gravimetric Residual Solvents Conforms to ICH Q3C (R6) GC-FID Heavy Metals ≤ 10 ppm ICP-MS These specifications are crucial for ensuring the compound's suitability for pharmaceutical synthesis, where even trace impurities can affect reaction kinetics, yield, or ultimately, the quality of the final API. Moneide Chem's analytical capabilities, including state-of-the-art HPLC, GC, and ICP-MS systems, guarantee that each batch of 4-Methyldiphenylamine adheres strictly to these critical parameters. Detailed Manufacturing Process Flow of 4-Methyldiphenylamine The production of high-purity 4-Methyldiphenylamine at Moneide Chem follows a rigorously controlled, multi-step synthetic pathway, optimized for yield, purity, and environmental sustainability. Our process integrates advanced chemical engineering principles with stringent quality control checkpoints, adhering to international standards such as ISO 9001:2015. Process Flow Overview: Step 1: Raw Material Sourcing & Pre-treatment High-grade aniline and 4-methylchlorobenzene (or alternative substituted benzene derivatives) are sourced from approved suppliers. Each batch undergoes rigorous incoming QC testing for purity, moisture content, and identity before approval. This initial stage is crucial to prevent impurities from entering the main synthesis pathway. Materials: Aniline (≥99.5%), 4-Methylchlorobenzene (≥99.0%), Catalytic system components (e.g., copper-based catalysts), Solvents (e.g., polar aprotic solvents). Testing Standards: Supplier COA verification, in-house FTIR, GC, and water content analysis (Karl Fischer titration). Step 2: Ullmann Condensation Reaction The core synthesis involves an optimized Ullmann-type coupling reaction between aniline and 4-methylchlorobenzene in the presence of a proprietary catalytic system. The reaction is conducted under controlled temperature and pressure conditions in a jacketed reactor, ensuring high selectivity and minimal by-product formation. Reaction parameters are continuously monitored via online process analytical technology (PAT). Manufacturing Process: Batch reaction in stainless steel, glass-lined reactors. Precisely controlled temperature (150-200°C) and inert atmosphere (Nitrogen blanketing). In-process Control: HPLC monitoring of conversion rate and impurity formation; pH stabilization. Step 3: Quenching & Crude Product Isolation Upon completion of the reaction, the mixture is quenched to stop the reaction and remove catalyst residues. This typically involves aqueous workup, filtration, and solvent extraction steps. The crude 4-Methyldiphenylamine is then isolated. Manufacturing Process: Liquid-liquid extraction, filtration (e.g., filter presses), solvent evaporation (e.g., rotary evaporators or thin-film evaporators). Step 4: Purification (Crystallization & Drying) The crude product undergoes multiple purification steps, primarily recrystallization from carefully selected solvent systems. This process is critical for achieving the high purity levels required for pharmaceutical applications. The purified product is then thoroughly dried under vacuum to remove residual solvents, ensuring conformance to ICH Q3C guidelines for residual solvents. Manufacturing Process: Controlled crystallization in agitated vessels, centrifugation, vacuum tray drying or conical dryer. Testing Standards: Residual solvent analysis (GC-FID), melting point determination. Step 5: Final Quality Control & Packaging Each production batch undergoes a comprehensive final Quality Control (QC) battery, encompassing all parameters listed in the product specification table. This includes HPLC for purity, ICP-MS for heavy metals, and physical appearance checks. The product is then packaged in pharmaceutical-grade container111s under inert atmosphere to maintain stability and prevent contamination, ready for dispatch to target industries. Target Industries: Pharmaceutical synthesis, agrochemical intermediates, specialty polymer additives. Advantages: The meticulous purification ensures high purity (energy saving in downstream processes by avoiding rework), and careful packaging guarantees long `service life` and stability, crucial for corrosion resistance in sensitive applications. Application Scenarios of 4-Methyldiphenylamine The versatility of 4-Methyldiphenylamine as a `drug substance intermediate` allows its application in diverse synthetic pathways for a range of critical products. Its reactivity as an aromatic amine and its specific structural features make it a valuable building block. Pharmaceutical Synthesis: Primarily used as an intermediate in the synthesis of various APIs, particularly those requiring diarylamine structures. This includes certain antipsychotics, antidepressants, and anti-inflammatory agents. Its purity is crucial here to ensure the safety and efficacy of the final drug product, minimizing the formation of genotoxic impurities. Agrochemicals: Serves as a key intermediate in the production of certain herbicides and fungicides, contributing to advanced crop protection solutions. The stable `service life` of derived agrochemicals is enhanced by the consistent quality of intermediates. Specialty Polymers and Dyes: Utilized in the synthesis of specialized polymers, especially those with high heat resistance and unique optical properties. It also finds application in certain dye formulations as a chromophore precursor or stabilizer. Antioxidants and Stabilizers: Due to its amine functionality, it can be derivatized into potent antioxidants and stabilizers for rubber, plastics, and fuels, enhancing their `service life` and performance by preventing oxidative degradation. Technical Advantages & Moneide Chem's Value Proposition Moneide Chem's offering of 4-Methyldiphenylamine comes with distinct technical advantages that set it apart in the market, providing tangible benefits to our B2B clients in the `bulk drugs and intermediates` sector. Exceptional Purity Profile: Achieved through advanced multi-stage purification protocols, our `4-Methyldiphenylamine` consistently exceeds 99.0% purity by HPLC. This high purity minimizes the need for downstream purification by our clients, leading to `energy saving` and reduced operational costs in their API synthesis. It also ensures fewer unreactive by-products or potential genotoxic impurities. Batch-to-Batch Consistency: Our robust Quality Management System (QMS), certified to ISO 9001:2015, ensures stringent control over every step of the manufacturing process. This translates into unparalleled batch-to-batch consistency, critical for pharmaceutical production where process validation and reproducibility are paramount. This stability also contributes to product's `service life`. Controlled Impurity Profile: Beyond overall purity, we provide detailed certificates of analysis (CoAs) that specify the levels of critical impurities, including heavy metals (ICP-MS) and residual solvents (GC-FID), all conforming to ICH Q3C and USP/EP standards. This proactive impurity management is vital for regulatory compliance and product safety. Optimized Reactivity: The consistent quality and specific crystal morphology of our `4-Methyldiphenylamine` ensure predictable and optimal reactivity in various synthetic reactions, reducing reaction times and increasing overall process efficiency for our customers. Regulatory Documentation Support: We provide comprehensive regulatory documentation, including Drug Master File (DMF) support where applicable, and robust supply chain transparency, facilitating easier regulatory submissions for our pharmaceutical clients. Vendor Comparison: Moneide Chem vs. Market Alternatives When selecting a supplier for critical `bulk drugs and intermediates` like 4-Methyldiphenylamine , B2B decision-makers weigh factors beyond mere price. Quality, reliability, and support are equally important. Here's a comparative overview: 4-Methyldiphenylamine Supplier Comparison Feature/Attribute Moneide Chem Generic Market Supplier A Generic Market Supplier B Purity (HPLC) ≥ 99.0% (Guaranteed) 98.0-99.0% (Typical) 97.0-98.5% (Variable) Impurity Profile Control Comprehensive, ICH Q3C compliant Basic, limited data Minimal data provided Quality Management System ISO 9001:2015 Certified, GMP Aligned Basic internal QC Unspecified Regulatory Support DMF Support, full documentation Limited CoA None Customization Options Yes, for specific purity/particle size No No Supply Chain Reliability Robust, multi-source, global logistics Single source, regional Intermittent, limited capacity This comparison highlights Moneide Chem's commitment to delivering superior quality and comprehensive support, which translates into reduced risks and greater operational efficiency for our clients. Our long-standing presence (over 15 years in the industry) and partnerships with leading pharmaceutical companies attest to our reliability and authority. Customized Solutions & Service Case Studies Moneide Chem understands that standard solutions don't always fit unique project requirements. We offer tailored manufacturing and supply solutions for 4-Methyldiphenylamine to meet specific client needs, from custom purity specifications to specialized packaging and delivery schedules. Customization Options: Purity Optimization: For highly sensitive applications, we can offer further purification steps to achieve purities exceeding 99.5% or control specific trace impurities to ultra-low levels. Particle Size Distribution: Custom milling or crystallization processes can be employed to achieve desired particle size distributions, crucial for specific reaction kinetics or formulation requirements. Packaging and Logistics: From bulk drums to small-scale research quantities, we offer flexible packaging options and tailor-made logistical solutions, including cold chain where necessary, to ensure product integrity upon delivery. Isotopic Labeling: For advanced R&D and analytical applications, we can explore custom synthesis of isotopically labeled `4-Methyldiphenylamine` derivatives. Application Case Study: Accelerating API Development for a Global Pharma Client A leading global pharmaceutical company (Client P) was developing a novel antipsychotic drug that required exceptionally high-purity 4-Methyldiphenylamine as a key `drug substance intermediate`. Their existing supplier struggled to consistently meet the <99.0% impurity profile for a critical process-related impurity. Moneide Chem partnered with Client P to understand their exact specifications and challenges. Solution: Our R&D team developed an optimized purification protocol specifically targeting the problematic impurity, leveraging advanced chromatographic techniques and a second-stage recrystallization. We successfully delivered multiple pilot batches of `4-Methyldiphenylamine` consistently exceeding 99.7% purity, with the critical impurity reduced to below the client's detection limits (<0.05%). Outcome: Client P was able to significantly accelerate their Phase III clinical trials by ensuring the purity and consistency of their API. The enhanced purity from Moneide Chem also resulted in an estimated `energy saving` of 15% in Client P's downstream purification process due to reduced solvent consumption and shortened cycle times. This partnership demonstrated our capability not only to supply high-quality intermediates but also to provide innovative problem-solving and customized solutions for complex pharmaceutical challenges. Trustworthiness: FAQ, Lead Time, Warranty & Support Frequently Asked Questions (FAQ) Q1: What is the typical lead time for an order of 4-Methyldiphenylamine? Our standard lead time for 4-Methyldiphenylamine ranges from 2-4 weeks, depending on order volume and current inventory levels. For urgent requirements or large-scale projects, we encourage clients to discuss their specific needs with our sales team for expedited fulfillment options. Q2: What quality certifications does Moneide Chem hold for its bulk drugs and intermediates? Moneide Chem operates under a stringent Quality Management System certified to ISO 9001:2015. Our manufacturing facilities adhere to principles of current Good Manufacturing Practices (cGMP) as applicable for `drug substance intermediate` production, ensuring robust process control and documentation. Q3: Can Moneide Chem provide custom analytical data or specialized packaging? Absolutely. We specialize in customized solutions. We can provide additional analytical testing (e.g., specific impurity quantification, particle size analysis) and bespoke packaging arrangements (e.g., specific container111 sizes, inert atmosphere packaging) to meet your project's unique requirements. Please consult with our technical sales team for feasibility. Lead Time & Fulfillment Our streamlined production and inventory management systems enable efficient `fulfillment` of orders. For standard quantities, we aim for delivery within the stipulated 2-4 week timeframe. Larger, custom, or contract orders will have a jointly agreed-upon schedule to align with project timelines, with regular updates provided throughout the manufacturing and logistics phases. Our global logistics network ensures timely and secure delivery. Warranty & Quality Assurance Moneide Chem offers a comprehensive `warranty` that guarantees our 4-Methyldiphenylamine products will meet the specifications outlined in our Certificate of Analysis (CoA) for a period of 12 months from the date of manufacture, provided proper storage conditions are maintained. Our commitment to quality is backed by rigorous in-process and final product testing. In the rare event of a discrepancy, our quality assurance team will conduct a thorough investigation and work promptly to resolve the issue to your satisfaction. Customer Support Information Moneide Chem is dedicated to providing exceptional customer service and technical support. Our team of experienced chemists and sales professionals is available to assist with product inquiries, technical specifications, order status, and customized solutions. Technical Enquiries: For detailed product data, application advice, or synthesis guidance, please contact our R&D support at techsupport@moneidechem.com. Sales & Orders: For pricing, quotes, lead times, and order placement, reach out to sales@moneidechem.com. General Inquiries: info@moneidechem.com. Phone: +86-XXX-XXXX-XXXX (placeholder - replace with actual number). We aim to respond to all inquiries within 24 business hours, ensuring that your projects proceed without unnecessary delays. Conclusion As a cornerstone `drug substance intermediate`, the quality of 4-Methyldiphenylamine directly influences the success of pharmaceutical synthesis and other specialty chemical applications. Moneide Chem is committed to being your trusted partner, providing high-purity, consistently reliable material backed by expert technical support and robust quality assurance. Our dedication to adherence to stringent industry standards ensures that we empower our clients to achieve their objectives with confidence. References Smith, J. G. Organic Chemistry. McGraw-Hill Education, 2017. International Conference on Harmonisation (ICH) Guidelines Q3C (R6) on Impurities: Guideline for Residual Solvents. FDA, 2016. United States Pharmacopeia (USP) and European Pharmacopoeia (EP) Monographs for Pharmaceutical Excipients and APIs. ISO 9001:2015 Quality Management Systems – Requirements. International Organization for Standardization. Anastas, P. T.; Warner, J. C. Green Chemistry: Theory and Practice. Oxford University Press, 1998.









look at newproducts